
Welcome to Leader in Water- & Wastewater Solution
Welcome to Leader in Water- & Wastewater Solution
The refrigeration process is used in gas plants to remove heat from certain process streams. Refrigeration in natural gas treating is a process that serves a dual dew point control function—namely, it is used to meet the hydrocarbon dew point as well as the water dew point specification for residue or sales gas.
The temperature to which the gas is cooled depends first on meeting these dew point specifications. This is the minimum cooling requirement. Cooling the gas to lower temperatures than the minimum temperature for dew point control has to be justified by the economics of liquefied petroleum gas (LPG) recovery.
This requires a cost analysis of the value of additional LPG recovery versus increased capital and operating costs. Additional recovery of LPG is achieved by either of the following methods:
• Chilling the gas to colder temperatures, such as –20 to –30°F
• Contacting the gas stream with lean oil in an absorption tower
Refrigeration is basically pumping heat from one medium to another. Heat by itself can only flow from a higher temperature medium to a lower temperature medium. Thus, refrigeration is a process that provides the cooling medium to which the gas is exposed. Refrigeration systems generally operate trouble free but can drop in efficiency, which requires investigation.
Fig. 1 shows the typical refrigeration equipment for natural gas cooling. The heat exchanger cools the incoming gas to the refrigeration unit by exchanging heat with the cold gas, which has been chilled to the design cold temperature in the propane chiller.
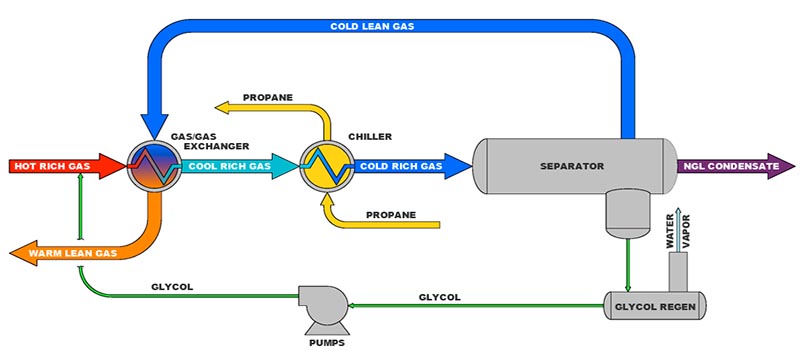
Because the gas entering the refrigeration unit is normally saturated with water vapor and the temperature to which the gas is cooled is substantially below the hydrate point of the gas, some means of preventing hydrate formation must be instituted. The formation temperature of hydrates at a given pressure can be suppressed by the addition of chemicals, such as methanol or glycol.
In conventional refrigeration units Propane, or some other refrigerant, boils in the chiller at a very low, controlled temperature, removing heat from the gas stream, thereby condensing a portion of the gas. The cold gas, condensate, and glycol flow from the chiller to a three-phase separator. The condensate goes to a fractionation unit. The gas is sufficiently cooled so it meets both the hydrocarbon and water dew points. It exchanges heat with the incoming gas to the refrigeration process.
The rich glycol is separated from the hydrocarbon gas stream in a three-phase separator and is routed to a regenerator. The concentration of the regenerated glycol is usually on the order of 75 to 80% glycol, with the balance being water. Sufficient glycol is injected at the two injection points to result in a mixture of water and glycol to depress the hydrate temperature to the required level, which for design purposes is the refrigerant boiling temperature.
The refrigeration effect is brought about by the vaporization of a refrigerant, such as propane, in the chiller. Propane is suited for this application, as it boils at temperatures near and below ambient temperatures. The vaporization of propane or boiling requires heat to effect the phase change from liquid to vapor—namely the latent heat of vaporization. By controlling the pressure at which the boiling of the propane takes place, the desired refrigeration temperature, down to about –40°F, is achieved.