
Welcome to Leader in Water- & Wastewater Solution
Welcome to Leader in Water- & Wastewater Solution
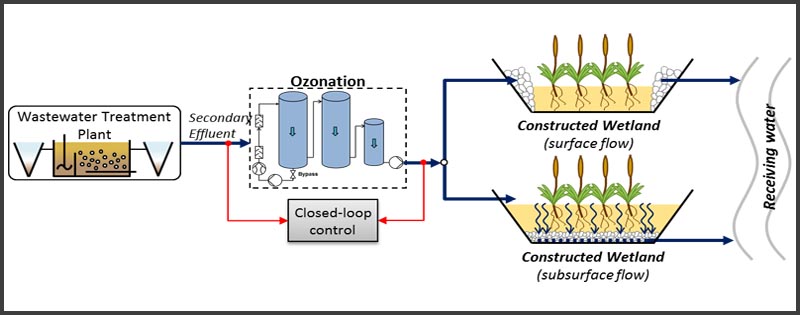
Disinfection is considered to be the primary mechanism for the inactivation/destruction of pathogenic organisms to prevent the spread of waterborne diseases to downstream users and the environment.
Ozone has been used for many years in drinking and waste water treatment plants throughout the world for sterilization and purification of water. Ozone is a very reactive form of oxygen that can destroy an enormous variety of liquid waste materials and toxins.
Ozone is produced when oxygen (O2) molecules are dissociated by an energy source into oxygen atoms and subsequently collide with an oxygen molecule to form an unstable gas, ozone (O3), which is used to disinfect water and wastewater. Ozone is generated onsite because it is unstable and decomposes to elemental oxygen in a short amount of time after generation.
When ozone decomposes in water, the free radicals hydrogen peroxy (HO2 ) and hydroxyl (OH) that are formed have great oxidizing capacity and play an active role in the disinfection process. It is generally believed that the bacteria are destroyed because of protoplasmic oxidation resulting in cell wall disintegration.