
Welcome to Leader in Water- & Wastewater Solution
Welcome to Leader in Water- & Wastewater Solution

High purity water production has traditionally used a combination of membrane separation and ion exchange processes. EDI is a process which combines semi-impermeable membrane technology with ion-exchange media to provide a high efficiency demineralization process. Electro dialysis employ electrical current and specially-prepared membranes which are semi permeable to ions based on their charge, electrical current, and ability to reduce the ions based to their charge. Through electro dialysis an electrical potential transports and segregates charged aqueous species. The electrical current is used to continuously regenerate the resin, eliminating the need for periodical regeneration.
The EDI process produces industrial process water of very high purity, using less than 95% of the chemical products used in the conventional ion exchange processes. With EDI system membranes and electricity replace the million gallons of acid and caustic chemicals that the old processes required daily.
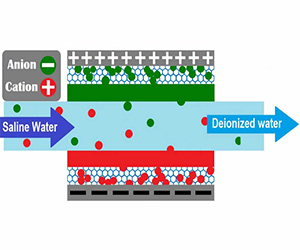
An EDI stack has the basic structure of a De ionization chamber. The chamber contains a ion exchange resin, packed between a cationic exchange membrane and a anionic exchange membrane. Only the ions can pass through the membrane, the water is blocked.
When flow enters the resin filled diluting compartment, several processes are set in motion. Strong ions are scavenged out of the feed stream by the mixed bed resins. Under the influence of the strong direct current field applied across the stack of components, charged ions are pulled off the resin and drawn towards the respective, oppositely-charged electrodes. In this way these charged strong-ion species are continuously removed and transferred in to the adiacent concentrating compartments. As the ions go towards the membrane, they can pass through the concentration chamber (see figure) but they cannot reach the electrode. They are blocked by the contiguous membrane, that contains a resin with the same charge.
As the strong ions are removed from the process stream, the conductivity of the stream becomes quite low. The strong, applied electrical potential splits water at the surface of the resin beads, producing hydrogen and hydroxyl ions. These act as continuous regenerating agents of the ion-exchange resin. These regenerated resins allow ionization of neutral or weakly-ionized aqueous species such as carbon dioxide or silica. Ionization is followed by removal through the direct current and the ion exchange membranes.
The ionization reactions occurring in the resin in hydrogen or hydroxide forms for the removal of weakly ionized compounds are listed below
EDI is useful for any application that requires constant and economic removal of water impurities without using dangerous chemical. Some examples are:
Since installation EDI units perform quite reliably, providing the customers with high purity production water for either power plant boiler feed or microchip rinse water. The water produced has met or exceeded customer high-purity water specifications. In addition, when a diluite stream cleaning was required as result of fouling, product quality was completely recovered.
As a substitute for the more traditional ion-exchange process, EDI brings advances in both energy and operating expenses to the high purity water treatment train. By eliminating the periodic regeneration requirement of ion exchange resin, environmental benefits are also realized by avoiding the handling and processing of acid and caustic chemicals brought to the site. Some of the advantages of the EDI as opposed to the conventional systems of ionic interchange are:
EDI cannot be used for water having hardness higher than 1, since the calcium carbonate would create a scab in the camera of the concentrated one, limiting the operation. It requires purification pretreatment
Carbon Dioxide will freely pass through an RO membrane, dissociating and raising the conductivity of water. Any ionic species formed from the carbon dioxide gas will lower the outlet resistivity of the water produced by EDI. The management of CO2 in water is typically handled in one or two ways: the pH of the water can be adjusted to allow the RO membrane to reject the ionic species or the carbon dioxide can be removed from the water using a strip gas.